The purpose of the featherweight machine is to allow patients to conduct Dialysis on their time day or night and while on the go so it is less impactful to their lives. AWAK Technologies a Singapore-based medical technology company recently received FDA Breakthrough Device designation for their wearable and portable dialysis device.

Pd Based Wak Of Awak Technologies Singapore The First Wak To Be Download Scientific Diagram
The Automated Wearable Artificial Kidney AWAK is based on tidal.

. The dialysis machine mixes and monitors the dialysate. With AWAK it allows them to receive dialysis from the comfort of their homes and in turn will also lighten the load on their caregivers who usually need to assist and accompany the patients to the clinic. The company was certified by SGS for ISO 13485 Quality Management System in September 2008 with the scope of designing manufacturing and distributing medical devices.
These innovative modalities the automated wearable artificial kidney AWAK wearable artificial kidney WAK and the implantable artificial kidney IAK aim to decrease the burden on patients their families and the healthcare system. The AWAK Peritoneal Dialysis machine allows Chronic Kidney Disease patients to avoid long hours of connection to traditional Dialysis Machines see video below. A world-first the AWAK PD device disrupts the mode of delivery in which peritoneal dialysis is currently administered.
Explains how AWAKs revolutionary technology is helping to adapt therapy to a patients lifestyle and thus changing dialysis forever. A world-first the AWAK PD device disrupts the mode of delivery in which peritoneal dialysis is currently administered. Nausea vomiting swelling and exhaustion are all possible symptoms.
The device allows dialysis to be performed on-the-go overcoming the. The new enables dialysis to be performed on-the-go helping to address the challenge of long hours of therapy and connection to large-size dialysis machines currently faced by renal patients. Founded in 2007 Private Company AWAK Technologies develop wearable dialysis machines 1kg and battery-operated for continuous dialysis.
AWAK requires significantly lesser amounts of liquids as compared to traditional home dialysis devices. Singapore-based AWAK bags 40m to bring wearable dialysis device to market Singapore healthtech firm Automated Wearable Artificial Kidney AWAK Technologies has raised US40 million in an. The device allows dialysis to be performed on-the-go overcoming the.
AWAK Technologies was incorporated in 2007 with offices in Singapore and Burbank CA and dedicated to the research development and marketing of sorbent-based kidney dialysis machine for the treatment of patients with end-stage renal disease. Currently HD and PD are tethered by large machinery significant power usage and large water requirement. Despite its smaller size compared to regular PD machines AWAKs device can be used for seven to 10 hours a day.
Even if you dont have any of these symptoms you could have a high amount of wastes in your blood that are potentially hazardous to your body. Despite its smaller size compared to regular PD machines AWAKs device can be used for seven to 10 hours a day. AWAK VIDEO - 2018.
It only holds 250 millimeters of fluid but the cartridge can clean the toxin-laden. January 11 2019. It only holds 250 millimetres of fluid but the cartridge can clean the toxin-laden.
The company was certified by SGS for ISO 13485 Quality Management System in September 2008 with the scope of designing manufacturing and distributing medical devices. This often occurs when just 10 to 15 of your renal function remains. AWAK Technologies develop wearable dialysis machines 1kg and battery-operated for continuous dialysis.
Called the AWAK Peritoneal Dialysis device or AWAK PD the technology uses AWAKs patented sorbent technology and offers a convenient means of dialysis for. Awak peritoneal dialysis AWAK PD device is a wearable and ultra-portable PD system which integrates the patented sorbent technology of the company.

Awak Technologies Home Facebook
Awak Automated Wearable Artificial Kidney Renal Replacement Innovation Corp

Awak Technologies Home Facebook

Baxter Enrolls First Patient In Home Hemodialysis Machine Us Trial

Global Bioartificial Kidney Market Is Estimated To Grow At A Cagr Of 36 87 Over The Forecast Period 2022 2030
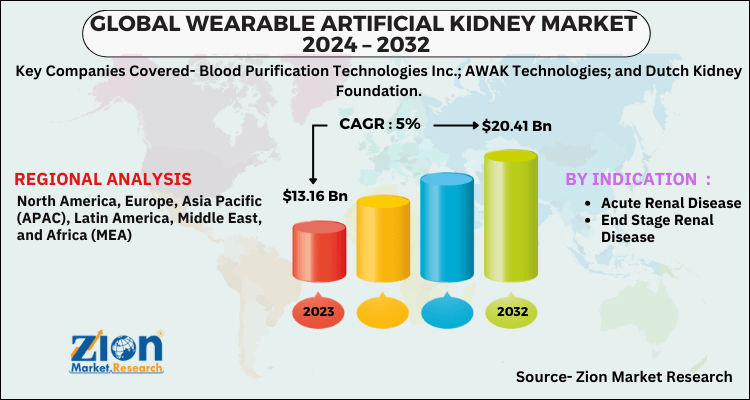
Global Wearable Artificial Kidney Market Set For Rapid Growth To Reach Value Around Usd 10 7 Billion By 2025 Ips Inter Press Service Business

Major Step Towards Portable Artificial Kidney

Sun 069 Evaluation Of Safety Of Automated Wearable Artificial Kidney Awak Device In Peritoneal Dialysis Patients Kidney International Reports

Awak Technologies Has A Sustainable Solution For Those Living With Kidney Disease An Artificial Kidney Device That Dialysis Peritoneal Dialysis Renal Disease

National Research University Of Electronic Technology Ppt Download
Awak Technologies Raises Us 40 Million To Ready Awak Pd

Awak In Southchina Morning Post Awak

Sun 069 Evaluation Of Safety Of Automated Wearable Artificial Kidney Awak Device In Peritoneal Dialysis Patients Kidney International Reports

Pd Based Wak Of Awak Technologies Singapore The First Wak To Be Download Scientific Diagram




